ISSUE1713
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Cynthia Covey has disclosed no relevant financial relationships.
- Review the efficacy and safety of Capvaxive, a 21-valent pneumococcal conjugate vaccine, for prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults.
- Description: A 21-valent pneumococcal conjugate vaccine (PCV21).
- Indication: Prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults.
- Coverage: PCV21 includes serotypes that collectively account for 85% of IPD cases in adults ≥65 years old.
- Adverse Effects: Injection-site reactions, fatigue, headache, myalgia, and arthralgia.
- Dosage: Single 0.5-mL dose given by intramuscular injection.
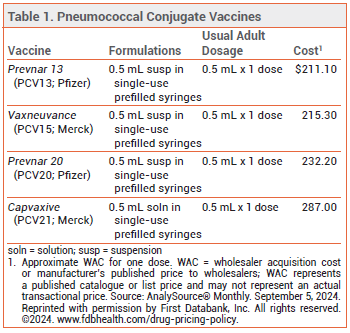
- Cost: One dose costs $287.
- Conclusion: The CDC Advisory Committee on Immunization Practices (ACIP) recommends PCV21 as an option for adults when a PCV vaccine is recommended.
Outline
Tables |
The FDA has licensed Capvaxive (PCV21; Merck), a 21-valent pneumococcal conjugate vaccine, for prevention of invasive pneumococcal disease (IPD) and pneumococcal pneumonia in adults. Four other pneumococcal vaccines are currently available in the US: Prevnar 20 (PCV20), Vaxneuvance (PCV15), and Prevnar 13 (PCV13) are conjugate vaccines licensed for use in persons ≥6 weeks old, and Pneumovax 23 (PPSV23) is a pneumococcal polysaccharide vaccine licensed for use in persons ≥2 years old (see Table 1).1

PNEUMOCOCCAL DISEASE ― Streptococcus pneumoniae is a common cause of bacterial pneumonia. Adults ≥65 years old and persons with certain medical and immunocompromising conditions (see Figure 1) are at increased risk of developing IPD. In the US, vaccination against S. pneumoniae has substantially reduced the incidence of pneumococcal disease in children and adults.
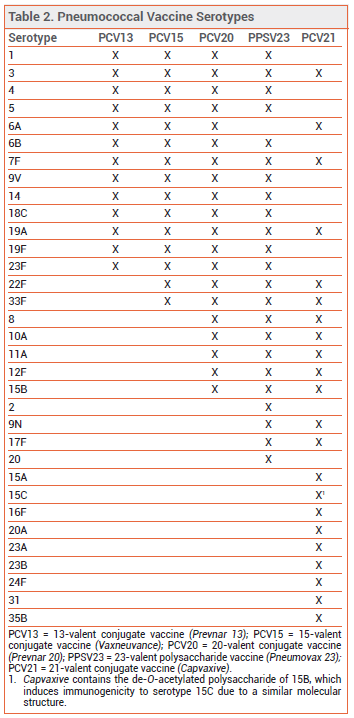
THE NEW VACCINE ― Like PCV13, PCV15, and PCV20, PCV21 contains capsular polysaccharide antigens conjugated to a protein carrier (nontoxic diphtheria CRM197 toxin). The 21 serotypes included in Capvaxive account for 85% of IPD cases in adults ≥65 years old; PCV20 includes serotypes that account for 54% of these cases. PCV21 includes 8 serotypes that are not included in any of the other currently available pneumococcal vaccines (see Table 2); these serotypes account for 30% of IPD cases in adults ≥65 years old. Some serotypes included in the other vaccines were excluded from PCV21 because they are no longer commonly associated with pneumococcal disease in adults.
Capvaxive does not protect against serotype 4, which is included in other pneumococcal vaccines and is responsible for ≥30% of IPD cases in certain populations (i.e., adults <65 years old living in some western US regions who are homeless, have chronic lung disease or alcohol use disorder, smoke, or use injectable drugs).
CLINICAL STUDIES ― FDA approval of Capvaxive was based on the results of 3 trials (STRIDE-3, STRIDE-5, and STRIDE-6; STRIDE-5 is unpublished) evaluating its immunogenicity in both vaccine-naive and previously vaccinated adults. In STRIDE-3 and STRIDE-6, immune responses elicited by PCV21 were noninferior to those elicited by PCV15, PCV20, and PPSV23 for all shared serotypes.2,3
In STRIDE-5 (summarized in the package insert), 1080 adults ≥50 years old were randomized to receive PCV21 with or 30 days after receiving a quadrivalent influenza vaccine. Immune responses elicited when the two vaccines were administered together were noninferior to those elicited when PCV21 was given 30 days after influenza vaccination for 20 of 21 pneumococcal serotypes (not serotype 23B) and for 3 of 4 influenza strains.
ADVERSE EFFECTS ― Injection-site reactions (pain, swelling, and erythema), fatigue, headache, myalgia, and arthralgia were the most common adverse effects reported with Capvaxive in clinical trials. Adverse effects were similar when Capvaxive was administered alone or with a quadrivalent inactivated influenza vaccine. Like other conjugate vaccines, Capvaxive is contraindicated for use in persons with a severe allergy to diphtheria toxoid.
PREGNANCY ― The American College of Obstetricians and Gynecologists (ACOG) recommends pneumococcal vaccination for pregnant women at increased risk of severe pneumococcal disease.4 No adequate data are available on the use of PCV21 in pregnant women. No adverse developmental outcomes were observed in animal studies.
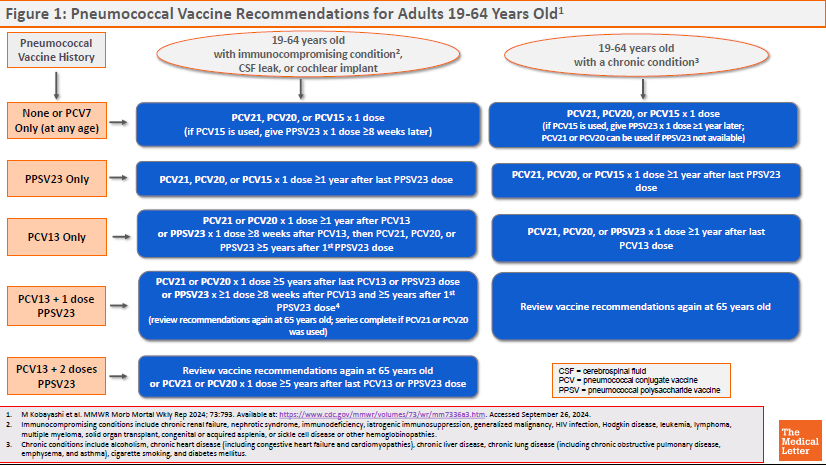
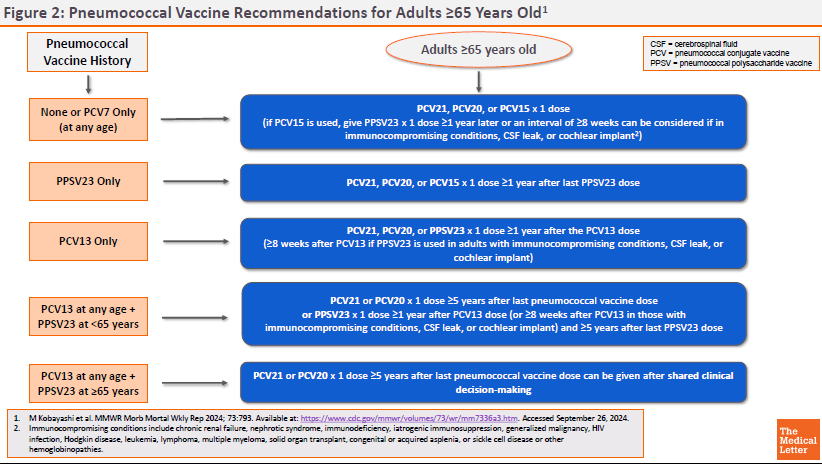
ACIP RECOMMENDATIONS ― The CDC Advisory Committee on Immunization Practices (ACIP) recommends PCV21 as an option for adults who are candidates for pneumococcal vaccination.5 Previously unvaccinated adults who are ≥65 years old or 19-64 years old with certain underlying medical conditions or other risk factors should receive a one-time dose of PCV21 or PCV20, or a dose of PCV15 followed ≥1 year later by PPSV23.
Specific recommendations for pneumococcal vaccination of adults based on vaccination status, age, and risk factors are available in Figures 1 and 2.
DOSAGE AND ADMINISTRATION ― Capvaxive is supplied in single-dose, prefilled syringes that should be stored in a refrigerator. It should be given intramuscularly as a single 0.5-mL dose.
CONCLUSION ― The new 21-valent pneumococcal conjugate vaccine Capvaxive contains serotypes that collectively account for 85% of invasive pneumococcal disease in older adults in the US, including 8 serotypes that are not included in other currently available pneumococcal vaccines. It is a recommended option for adults who are candidates for pneumococcal vaccination.
- Two new pneumococcal vaccines – Prevnar 20 and Vaxneuvance. Med Lett Drugs Ther 2021; 63:188.
- HL Platt et al. Safety, tolerability, and immunogenicity of an adult pneumococcal conjugate vaccine, V116 (STRIDE-3): a randomised, double-blind, active comparator controlled, international phase 3 trial. Lancet Infect Dis 2024 July 1 (epub). doi:10.1016/s1473-3099(24)00344-x
- P Scott et al. A phase 3 clinical study to evaluate the safety, tolerability, and immunogenicity of V116 in pneumococcal vaccine-experienced adults 50 years of age or older (Stride-6). Clin Infect Dis 2024 July 31 (epub). doi:10.1093/cid/ciae383
- American College of Obstetricians and Gynecologists. Maternal immunization. Practice advisory. October 2022. Available at: https://bit.ly/3ys9uyu. Accessed September 26, 2024.
- M Kobayashi et al. Use of 21-valent pneumococcal conjugate vaccine among U.S. adults: recommendations of the Advisory Committee on Immunization Practices - United States, 2024. MMWR Morb Mortal Wkly Rep 2024; 73:793. doi: 10.15585/mmwr.mm7336a3