ONLY
ARTICLE
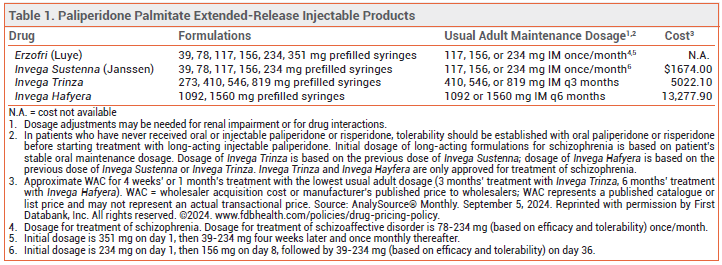
The FDA has approved Erzofri (Luye), an extended-release injectable formulation of the second-generation antipsychotic drug paliperidone palmitate, for treatment of schizophrenia and schizoaffective disorder in adults. It is the second once-monthly formulation of paliperidone palmitate to be approved in the US for these indications; Invega Sustenna was the first. Longer-acting injectable formulations of paliperidone palmitate are also available (see Table 1).1

LONG-ACTING INJECTABLE ANTIPSYCHOTICS ― Extended-release injectable second-generation antipsychotic drugs are generally used in patients with a history of relapse due to poor adherence to oral maintenance therapy.2
CLINICAL STUDIES ― No new clinical efficacy trials were required for FDA approval of Erzofri; approval was based on earlier trials with Invega Sustenna and an open-label study showing that the bioavailability of paliperidone palmitate with Erzofri was similar to that with Invega Sustenna.3
DOSAGE AND ADMINISTRATION ― The initial dose of Erzofri is 351 mg injected IM into the deltoid muscle; subsequent doses can be given in the gluteal or deltoid muscle. For schizophrenia, the recommended dosage is 117 mg IM (range 39-234 mg) once monthly. In patients with mild renal impairment, the recommended initial dose is 234 mg, followed by 78 mg once monthly (max 156 mg). Erzofri is not recommended for use in patients with moderate or severe renal impairment.
CONCLUSION ― A once-monthly, extended-release injectable formulation of the second-generation antipsychotic drug paliperidone palmitate (Invega Sustenna) has been available for years. Erzofri has not been shown to offer any advantage in efficacy or safety over Invega Sustenna. Extended-release formulations of paliperidone palmitate that are administered once every 3 months or 6 months are also available and may be preferred.
- In brief: Twice-yearly paliperidone (Invega Hafyera) for schizophrenia. Med Lett Drugs Ther 2022; 64:7.
- GA Keepers et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 2020; 177:868. doi:10.1176/appi.ajp.2020.177901
- ClinicalKey Clinical Trial. A study to evaluate the PK profiles of LY03010 and relative bioavailability at steady-state of LY03010 versus Invega Sustenna in schizophrenia patients. Available at: https://bit.ly/4gc2jLQ. Accessed September 12, 2024.