ISSUE1714
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Review the recommendations for use of the 2024-2025 Novavax COVID-19 vaccine.
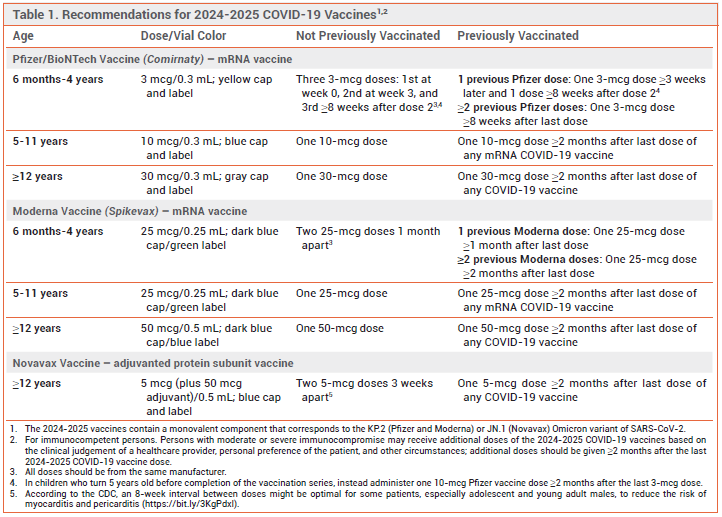
A 2024-2025 formulation of the Novavax adjuvanted protein subunit COVID-19 vaccine that more closely targets currently circulating SARS-CoV-2 variants is available now under an FDA Emergency Use Authorization (EUA) for use in persons ≥12 years old.1,2 The 2024-2025 formulations of the mRNA COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) were licensed by the FDA last month for use in persons ≥12 years old and made available under EUAs for use in persons 6 months to 11 years old.3
THE NEW FORMULATION — The 2024-2025 formulation of the Novavax vaccine contains the spike protein of the JN.1 Omicron strain of SARS-CoV- 2. Currently prevalent "FLiRT" variants (e.g., KP.2, KP.2.3, KP.3, KP.3.1.1, LB.1) are descended from the JN.1 strain.4,5 The Pfizer and Moderna 2024-2025 vaccines code for the spike protein of the KP.2 strain.3
EFFECTIVENESS — As with the mRNA COVID-19 2024-2025 vaccines, no clinical studies evaluating the immunogenicity or effectiveness of the 2024- 2025 Novavax vaccine are available. Authorization of the new formulation was based on the immunogenicity, safety, and efficacy of previous vaccine formulations.1,2
Observational studies suggest that all of the 2023-2024 formulations of the COVID-19 vaccines available in the US were effective in reducing the incidence of COVID-19, but data specific to the Novavax vaccine are limited. In a case-control analysis of 14,860 COVID-19 nucleic acid amplification tests administered at community pharmacies to immunocompetent adults with COVID-like symptoms between September 2023 and May 2024, receipt of any 2023-2024 COVID-19 vaccine at least 7 days before the test was associated with a decreased incidence of SARS-CoV-2 infection. The estimated adjusted vaccine efficacy was 45%; it was 58% for infections likely caused by the XBB.1.5 variant, which the 2023-2024 vaccines targeted, and 37% for infections likely caused by the JN.1 variant.6,7
In similar analyses of tests administered to adults with COVID-like illness within 10 days before or 3 days after an emergency department/urgent care visit (n=245,504) or a hospitalization (n=77,103) between September 2023 and May 2024, receipt of any 2023-2024 COVID-19 vaccine at least 7 days before the test was associated with a decreased incidence of COVID-19 requiring an emergency department/urgent care visit (estimated adjusted vaccine efficacy 36%) or hospitalization (estimated adjusted vaccine efficacy 42%). Among hospitalized immunocompetent persons, the estimated adjusted vaccine efficacy against critical illness was 58%.6,8
ADVERSE EFFECTS — Adverse effects of previous formulations of the Novavax vaccine have included injection-site pain/tenderness, fatigue/malaise, myalgia, arthralgia, headache, nausea, and vomiting. Hypersensitivity reactions, myocarditis, and pericarditis have occurred rarely; the incidence of myocarditis and pericarditis is highest in adolescent and young adult males.9
DOSAGE RECOMMENDATIONS — The 2024-2025 Novavax COVID-19 vaccine is indicated for use in persons ≥12 years old. Generally, persons who have not been vaccinated against COVID-19 previously should receive 2 doses 3-8 weeks apart. Those who have been vaccinated against COVID-19 previously should receive a single 0.5-mL dose ≥2 months after their last COVID-19 vaccine dose. Additional doses, each given ≥8 weeks after the previous dose, can be considered for persons with immunocompromise (solid-organ transplant recipients and equivalent).2,10
CDC RECOMMENDATIONS — The CDC recommends that all persons ≥6 months old be immunized with a 2024-2025 COVID-19 vaccine formulation. Persons ≥12 years old can receive a Pfizer, Moderna, or Novavax vaccine. Persons 6 months to 11 years old should receive a Pfizer or Moderna vaccine.10
- FDA News Release. FDA authorizes updated Novavax COVID-19 vaccine to better protect against currently circulating variants. August 30, 2024. Available at: https://bit.ly/4g8ZpY6. Accessed October 9, 2024.
- FDA. Fact sheet for healthcare providers administering vaccine: Emergency Use Authorization of Novavax COVID-19 vaccine, adjuvanted (2023-2024 formula), for individuals 12 years of age and older. August 2024. Available at: https://bit.ly/46M6kBa. Accessed October 9, 2024.
- COVID-19 update: New Pfizer and Moderna vaccine formulations for 2024-2025. Med Lett Drugs Ther 2024; 66:151.
- COVID-19 Real-Time Learning Network. COVID-19 variant update. September 17, 2024. Available at: https://bit.ly/3XslucR. Accessed October 9, 2024.
- CDC. COVID data tracker. Variant proportions. September 14, 2024. Available at: https://bit.ly/3Ka3HhH. Accessed October 9, 2024.
- R Link-Gelles. Effectiveness of COVID-19 (2023-2024 formula) vaccines. ACIP Meeting COVID-19 Vaccines. June 27, 2024. Available at: https://bit.ly/4gA1ixl. Accessed October 9, 2024.
- R Link-Gelles et al. Early estimates of updated 2023-2024 (monovalent XBB.1.5) COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infection attributable to co-circulating Omicron variants among immunocompetent adults - increasing community access to testing program, United States, September 2023-January 2024. MMWR Morb Mortal Wkly Rep 2024; 73:77. doi:10.15585/mmwr.mm7304a2
- J DeCuir et al. Interim effectiveness of updated 2023–2024 (monovalent XBB.1.5) COVID-19 vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalization among immunocompetent adults aged ≥18 Years — VISION and IVY Networks, September 2023–January 2024. MMWR Morb Mortal Wkly Rep 2024; 73:180. doi:10.15585/mmwr.mm7308a5
- CDC. Clinical considerations: myocarditis and pericarditis after receipt of COVID-19 vaccines among adolescents and young adults. October 10, 2023. Available at: https://bit.ly/3yXicot. Accessed October 9, 2024.
- CDC. Interim clinical considerations for use of COVID-19 vaccines in the United States. September 6, 2024. Available at: https://bit.ly/3KgPdxl. Accessed October 9, 2024.