ISSUE1730
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Understand recent regulatory and pharmaceutical developments, including the removal of REMS for clozapine and the approval of Omlyclo as a biosimilar interchangeable with Xolair, and explain their implications for clinical practice.
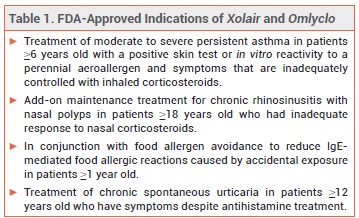
Omlyclo (omalizumab-igec; Celltrion), a biosimilar product interchangeable with the recombinant anti-IgE monoclonal antibody Xolair, has been approved by the FDA for same indications as Xolair (see Table 1). Omlyclo is the first Xolair biosimilar to be approved in the US.

REGULATORY STATUS — A biosimilar is a biologic product that is highly similar in composition and strength and has no clinically meaningful differences in safety, purity, and potency to the FDA-approved reference product. For a biosimilar to be approved as an interchangeable product, the manufacturer generally conducts clinical trials to prove that the results will be the same if the patient switches between the reference product and the biosimilar. In clinical studies, there were no clinically significant differences in efficacy, safety, and immunogenicity between Omlyclo and Xolair for treatment of chronic spontaneous urticaria. FDA approval of Omlyclo for allergic asthma, chronic rhinosinusitis with nasal polyps, and food allergic reactions was extrapolated based on available data.1-3
According to federal law, an interchangeable product can be substituted for the reference product by the pharmacist without permission from the prescriber. Some states require the pharmacist to notify the prescriber and/or patient before making the substitution; currently four states (AL, IN, SC, and WA) restrict interchangeability entirely.4
AVAILABILITY AND COST — Omlyclo will not be available until September 2026; Xolair's patent exclusivity expires in November 2025. The price of Omlyclo has not been announced but will presumably be less than that of Xolair. The wholesale acquisition cost (WAC) for a one-year supply of Xolair ranges from about $9000 to $144,000 for treatment of food allergic reactions.5
- SS Saini et al. CT-P39 compared with reference omalizumab in chronic spontaneous urticaria: results from a double-blind, randomized, active-controlled, phase 3 study. Allergy 2025 Jan 9 (epub). doi:10.1111/all.16446
- T Hasunuma et al. Pharmacokinetics and safety of CT-P39 via auto-injector are comparable to reference omalizumab via prefilled syringe. Immunotherapy 2025; 17:113. doi:10.1080/1750 743x.2025.2467026
- M Maurer et al. Pharmacokinetic equivalence of CT-P39 and reference omalizumab in healthy individuals: a randomised, double-blind, parallel-group, phase 1 trial. Clin Transl Allergy 2022; 12:e12204. doi:10.1002/clt2.12204
- S Humphreys. Understanding interchangeable biosimilars at the federal and state levels. Am J Manag Care 2023; 29(7 Spec No.):SP545. doi:10.37765/ajmc.2023.89419
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. May 5, 2025. Reprinted with permission by First Databank, Inc. All rights reserved. ©2025. www.fdbhealth.com/drug-pricing-policy.