ISSUE1743
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of oral semaglutide (Rybelsus) for cardiovascular risk reduction in patients with type 2 diabetes.
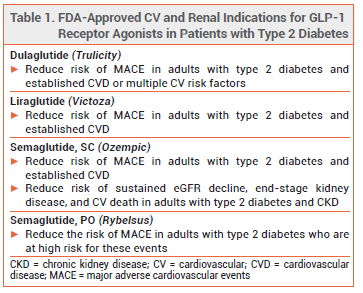
The oral glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Rybelsus – Novo Nordisk), which was approved by the FDA in 2019 for treatment of type 2 diabetes in adults, has now also been approved to reduce the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes who are at high risk for these events.1 The injectable GLP-1 receptor agonists semaglutide (Ozempic), dulaglutide (Trulicity), and liraglutide (Victoza) are also approved for cardiovascular risk reduction in patients with type 2 diabetes (see Table 1).2
CLINICAL STUDIES — FDA approval of oral semaglutide for the new indication was based on the results of a double-blind trial (SOUL) in 9650 patients ≥50 years old with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD), chronic kidney disease (CKD), or both. About 87% of patients had cardiovascular disease at enrollment. Patients were randomized to receive oral semaglutide titrated to 14 mg once daily or placebo in addition to standard care (other glucose-lowering and cardiovascular risk-reducing drugs). After a median of 49.5 months, the incidence of MACE (a composite of nonfatal myocardial infarction [MI], nonfatal stroke, or cardiovascular death), the primary endpoint, was statistically significantly lower in the semaglutide arm compared to the placebo arm (12.0% vs 13.8%; HR 0.86, 95% CI 0.77-0.96 [number needed to treat 55.6]). The reduction was primarily driven by a reduction in nonfatal MI (HR 0.74, 95% CI 0.61-0.89). Cardiovascular mortality and adverse kidney outcomes were similar in both groups.3
DOSAGE, ADMINISTRATION, AND COST — Rybelsus is available in two formulations (R1 and R2). The R1 formulation, which is available in 3-, 7-, and 14-mg tablets, should be used for the new indication. Slow titration of the dose can reduce GI adverse effects and improve tolerability. The recommended starting dosage is 3 mg once daily for 30 days; the dose should be increased to 7 mg once daily for 60 days and, if needed for glycemic control, to 14 mg once daily thereafter. All patients in the SOUL trial were titrated to a semaglutide dose of 14 mg. Rybelsus should be taken on an empty stomach in the morning with no more than 4 ounces of plain water. The wholesale acquisition cost (WAC) for a 30-day supply of Rybelsus is $997.60.4
CONCLUSION — Addition of the oral glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Rybelsus) to standard treatment reduced the risk of a major adverse cardiovascular event (MACE) in adults with type 2 diabetes and atherosclerotic cardiovascular disease and/or chronic kidney disease. Rybelsus has not been directly compared with injectable GLP-1 receptor agonists for this indication.
View the Comparison Table: GLP-1 and GIP/GLP-1 Receptor Agonists for Type 2 Diabetes
- Oral semaglutide (Rybelsus) for type 2 diabetes. Med Lett Drugs Ther 2019; 61:166.
- Non-insulin drugs for type 2 diabetes. Med Lett Drugs Ther 2025 in press.
- DK McGuire et al. Oral semaglutide and cardiovascular outcomes in high-risk type 2 diabetes. N Engl J Med 2025; 392:2001. doi:10.1056/nejmoa2501006
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. November 5, 2025. Reprinted with permission by First Databank, Inc. All rights reserved. ©2025. www.fdbhealth.com/policies/drug-pricing-policy.